Abstract
BACKGROUND: Our recent studies indicate that circulating PF4/heparin complexes activate complement and contribute to a subsequent immune response seen in heparin-induced thrombocytopenia (HIT) (Khandelwal, Blood 2016). We undertook studies to develop a sensitive assay for measuring circulating PF4/heparin. To do so, we utilize a quartz crystal microbalance (QCM) device, which is a piezoelectric acoustic resonator that detects decreases in the resonance frequency of the quartz crystal due to the increased mass caused by molecules binding to the electrode surface. We hypothesize that a novel label-free QCM biosensor functionalized with PF4/heparin capture antibodies can provide for robust quantitative antigen detection and physical characterization of antigen-antibody interactions.
METHODS: We formed a mixed self-assembled monolayer by using thiol-functionalized polyethylene glycols (PEGs) with -OH and biotin terminated head-groups on gold electrodes of quartz biosensor (Nelson; Langmuir 2001). PEG-backfilling of this surface provided protein non-fouling effects (1% BSA adsorbed only 10 ng/cm2), while biotin end-groups enabled close to full surface coverage of streptavidin (180 ng/cm2). This surface became our platform to immobilize biotinylated KKO (anti-PF4/heparin IgG2Κb monoclonal antibody, developed in Arepally Lab) and TRA (isotype IgG2Κb control antibody; developed in Arepally Lab).
To characterize the sensor's detection ability, we flowed recombinant PF4 (provided by M. Poncz, Children's Hospital of Philadelphia) with a range of heparin (Elkins-Sinn, NJ) concentrations. The solutions are introduced to the biosensors 10 minutes after mixing the heparin and flowed through the system until the frequency readout stabilized. We determined the amount of captured PF4/heparin complexes and the layer thickness by monitoring the sensor's resonance frequency shifts and acoustic dissipation.
The surface distribution of PF4/heparin complexes on KKO-functionalized surfaces was imaged by tapping-mode atomic force microscopy (AFM). The imaging results are used to verify layer thickness calculated by acoustic modeling of the QCM signal.
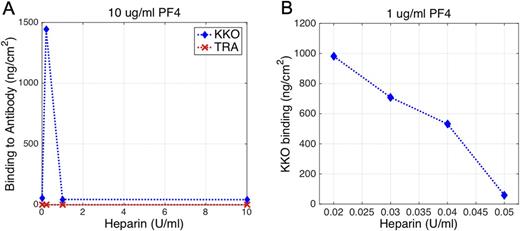
RESULTS: In initial experiments, KKO showed no changes in the presence of buffer or PF4 alone. However, when PF4 (10 μg/ml) was infused with increasing amounts of heparin (0.2, 1 or 10 U/ml) significant binding was detected starting at 0.2 U/ml heparin (Figure A). The acoustic modeling of the KKO-bound PF4 complexes with 0.2 U/ml heparin suggested that the macromolecular complexes were nearly 25 nm high. These results are consistent with the neutralization model for the role of PF4 in heparin-induced thrombocytopenia, in which the amount of PF4/ heparin complexes peaks at a specific molar ratio of PF4:heparin (Kowalska; Thrombosis Research 2010). Comparatively, almost no binding occurred when the quartz crystal was functionalized with TRA. This result not only demonstrates the protein non-fouling capacity of the PEG backfilling of the surface, but also shows the specificity of the surface-immobilized assay.
To gauge the sensitivity of the QCM biosensor to lower concentrations of PF4 and heparin that may be found in vivo, we tested 1µg/mL PF4 with lower heparin concentrations (0.02-0.05 U/ml). As shown in Figure B, a nearly linear KKO binding response was observed in this range capable of distinguishing heparin concentrations that differed by as little as 0.01 U/ml. The highest adsorption was observed at heparin concentration of 0.02 U/ml with associated atomic force microscopy (AFM) images again showing spherical particles with 25 nm diameter on the KKO immobilized surface.
CONCLUSIONS: Highly specific binding to PF4 complexes demonstrates the sensitivity of the QCM platform and its ability to distinguish differences between heparin concentrations of even 0.01 U/ml. Since QCM is a mass-sensing biosensor, it has a unique sensitivity advantage while sensing high molecular weight macromolecular complexes. This, combined with possible miniaturization of the sensor and integration with lab-on-a-chip systems, creates the potential for point-of-care bed-side detection of PF4/heparin.
Arepally: Biokit: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal